Multiple Choice
Find the volume of 0.110 mol L-1 hydrochloric acid necessary to react completely with 1.52 g 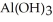 .
.
A) 0.658 L
B) 1.52 L
C) 1.88 L
D) 1.01 L
E) 0.531 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: Aluminum metal reacts with aqueous iron(II)chloride to
Q73: What is the oxidation number of the
Q88: Dinitrogen monoxide gas decomposes to form nitrogen
Q158: How many of the following compounds are
Q201: Which one of the following compounds is
Q202: Match the following.<br>-Mg(s) + Cu(NO<sub>3</sub>)<sub>2</sub>(aq) → Mg(NO<sub>3</sub>)<sub>2</sub>(aq)
Q203: A solution is prepared by adding 1.40
Q205: What is the concentration of an AlCl<sub>3</sub>
Q209: What volume (in mL) of a 0.0887
Q211: A homogeneous mixture of two substances is