Multiple Choice
Lithium and nitrogen react to produce lithium nitride: 6Li(s) + 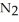 (g) →
(g) → 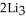 N(s)
N(s)
How many moles of lithium nitride are produced when 0.450 mol of lithium react in this fashion?
A) 0.150
B) 0.900
C) 0.0750
D) 1.35
E) 0.225
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: Balance the following redox reaction if it
Q81: In an acid-base neutralization reaction,38.74 mL of
Q230: Which of the following pairs of aqueous
Q231: How many litres of a 0.0550 mol
Q232: How many grams of NaCl are required
Q233: Automotive air bags inflate when sodium azide
Q234: What are the required coefficients to properly
Q236: Identify the balanced equation to show the
Q237: What are the required coefficients to properly
Q238: How many moles of NaCl are required