Multiple Choice
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) + 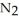 (g) →
(g) → 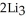 N(s)
N(s)
How many moles of lithium are needed to produce 0.40 mol of 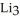 N when the reaction is carried out in the presence of excess nitrogen?
N when the reaction is carried out in the presence of excess nitrogen?
A) 0.13
B) 1.2
C) 0.20
D) 0.067
E) 2.4
Correct Answer:

Verified
Correct Answer:
Verified
Q14: A student dissolved 4.00 g of Co(NO<sub>3</sub>)<sub>2</sub>
Q91: How many moles of CuO can be
Q123: 5.0 g of iron is reacted with
Q157: What element is being oxidized in the
Q158: Which of the following is a gas-evolution
Q159: What is the concentration (M)of a NaCl
Q162: What mass (in g) of AgCl is
Q163: Which of the following is a weak
Q164: How many molecules of sucrose (C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>, molar
Q213: A 12.39 g sample of phosphorus reacts