Multiple Choice
Lithium and nitrogen react to produce lithium nitride: 6Li(s) + 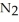 (g) →
(g) → 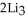 N(s)
N(s)
How many moles of 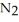 are needed to react with 0.400 mol of lithium?
are needed to react with 0.400 mol of lithium?
A) 2.40
B) 0.400
C) 0.133
D) 1.2
E) 0.0667
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: Based on the balanced chemical equation shown
Q225: Identify the balanced equation to show the
Q226: Lead ions can be precipitated from aqueous
Q227: How many moles of LiI are contained
Q228: A solution is prepared by mixing 70.0
Q230: Which of the following pairs of aqueous
Q231: How many litres of a 0.0550 mol
Q232: How many grams of NaCl are required
Q233: Automotive air bags inflate when sodium azide
Q234: What are the required coefficients to properly