Multiple Choice
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) +  (g) →
(g) → 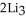 N(s)
N(s)
In a particular experiment, 5.50 g samples of each reagent are reacted. What is the theoretical yield of lithium nitride?
A) 4.60 g
B) 13.7 g
C) 27.6 g
D) 3.42 g
E) 9.20 g
Correct Answer:

Verified
Correct Answer:
Verified
Q168: If the reaction of phosphate ion with
Q191: Which of the following compounds is soluble
Q192: Determine the molarity of a solution formed
Q193: How many millilitres of a stock solution
Q195: Identify the percent yield when 28.16 g
Q197: Which of the following statements is TRUE?<br>A)
Q198: Balance the following equation:<br>_ C<sub>9</sub>H<sub>20</sub> + _
Q199: According to the following reaction, what mass
Q200: What are the required coefficients to properly
Q201: Which one of the following compounds is