Multiple Choice
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s) + 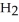 O(l) →
O(l) → 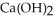 (s)
(s)
In a particular experiment, 5.00 g sample of CaO is reacted with excess water and 6.11 g of 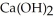 is recovered. What is the percent yield in this experiment?
is recovered. What is the percent yield in this experiment?
A) 81.8
B) 1.22
C) 108
D) 122
E) 92.5
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Based on the balanced chemical equation shown
Q65: A student prepared a stock solution by
Q71: How many grams of CH<sub>3</sub>OH must
Q131: Calcium phosphate reacts with sulfuric acid to
Q163: What precipitate is most likely formed from
Q216: Which of the following pairs of aqueous
Q217: Why is sugar water not a good
Q222: What element is being reduced in the
Q225: Identify the balanced equation to show the
Q226: Lead ions can be precipitated from aqueous