Multiple Choice
A stock solution of HNO3 is prepared and found to contain 13.7 mol L-1 of 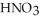 . If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, what is the concentration of the diluted solution?
. If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, what is the concentration of the diluted solution?
A) 1.46 mol L-1
B) 274 mol L-1
C) 0.274 mol L-1
D) 685 mol L-1
E) 0.685 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Which one of the following is the
Q22: How many millilitres of 0.260 mol L<sup>-1</sup>
Q27: How many millilitres of 0.152 mol L<sup>-1</sup>
Q28: Calculate the number of grams of solute
Q29: Determine the oxidation state of S in
Q30: Match the following.<br>-H<sub>2</sub>SO<sub>4</sub>(aq) + 2LiOH(aq) → 2H<sub>2</sub>O(l)
Q48: Carbonic acid can form water and carbon
Q98: Balance the following redox reaction if it
Q104: Balance the following redox reaction if it
Q136: Identify the location of oxidation in an