Multiple Choice
The chemical acidity of a solution is measured in units of pH: 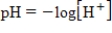 , where
, where 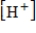 is the hydrogen ion concentration in the solution.If a sample of rain has a pH of 3.3, how many times higher is its
is the hydrogen ion concentration in the solution.If a sample of rain has a pH of 3.3, how many times higher is its 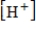 than pure water's, which has a pH of 7?
than pure water's, which has a pH of 7?
A) 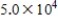
B) 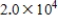
C) 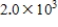
D) 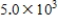
E) 7
Correct Answer:

Verified
Correct Answer:
Verified
Q139: Solve the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7896/.jpg" alt="Solve the
Q140: Select the graph of the function.
Q141: Condense the expression to the logarithm of
Q142: The pH of an acidic solution is
Q143: The populations P (in thousands) of Pittsburgh,
Q145: Rewrite the logarithm as a ratio of
Q146: The population P of a bacteria culture
Q147: Use the One-to-One Property to solve the
Q148: Solve for x. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7896/.jpg" alt="Solve
Q149: given that f(x) = ln x.<br><br>f(x -