Multiple Choice
Using the table of bond dissociation energies, the OH for the following gas- phase reaction is
KJ) 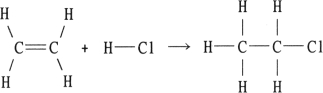
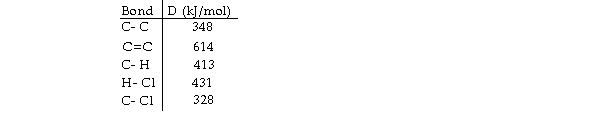
A) - 44
B) - 38
C) 304
D) 38
E) 2134
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: The strength of a covalent bond is
Q82: How many unpaired electrons are there in
Q83: The principal quantum number of the electrons
Q84: The ion NO<sup>-</sup><sup> </sup>has _ valence electrons.<br>A)
Q85: Of the atoms below, is the least
Q86: There are paired and unpaired electrons in
Q88: Which one of the following species has
Q89: The electron configuration of the phosph?ide ion
Q90: Which of the elements below has the
Q92: Why don't we draw double bonds between