Multiple Choice
The formal charge on sulfur in SO42- is , where the Lewis structure of the ion is: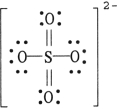
A) 0
B) - 4
C) - 2
D) +4
E) +2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: Of the atoms below, is the most
Q30: As the number of covalent bonds between
Q31: Of the bonds below, is the least
Q33: The electron configuration that corresponds to the
Q35: The type of compound that is most
Q36: Which of the following would have to
Q37: Of the bonds C- N, C=N, and
Q38: Using the table of average bond energies
Q39: The bond length in an HI molecule
Q87: As electronegativity difference increases,bond length will decrease.