Multiple Choice
The formal charge on carbon in the molecule below is .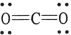
A) +1
B) +2
C) +3
D) - 1
E) 0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Determining lattice energy from Born- Haber cycle
Q8: A valid Lewis structure of _ _
Q9: In the nitrite ion (NO<sub>2</sub><sup>-</sup><sup> </sup>), .<br>A)
Q10: The Lewis structure of AsH<sub>3</sub><sub> </sub>shows nonbonding
Q11: The diagram below is the Born- huber
Q13: Lattice energy is .<br>A) the energy required
Q14: The Lewis structure of N<sub>2</sub>H<sub>2</sub><sub> </sub>shows .<br>A)
Q81: Alternative but equivalent Lewis structures are called
Q92: How many hydrogen atoms must bond to
Q127: When a metal gains an electron,the process