Multiple Choice
Using the table of average bond energies below, the OH for the reaction is kJ.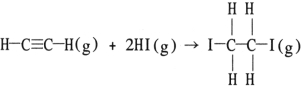

A) - 160
B) - 217
C) +160
D) - 63
E) +63
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q52: Which two bonds are most similar in
Q53: A valid Lewis structure of _ _
Q54: Which halogen, bromine or iodine, will form
Q55: Dynamite consists of nitroglycerine mixed with .<br>A)
Q58: Which ion below has a noble gas
Q59: How many equivalent resonance forms can be
Q60: Give the electron configuration of Cu<sup>2</sup><sup>+</sup>.
Q61: The central atom in _ _ does
Q84: Atoms surrounded by eight valence electrons tend
Q117: Calculate the bond energy of C-F given