Multiple Choice
In the resonance form of ozone shown below, the formal charge on the central oxygen atom is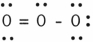
A) +1
B) +2
C) 0
D) - 2
E) - 1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: The strength of a covalent bond is
Q82: Draw the Lewis structure of ICl<sub>2</sub><sup>+</sup>.
Q88: Which one of the following species has
Q89: The electron configuration of the phosph?ide ion
Q90: Which of the elements below has the
Q92: Why don't we draw double bonds between
Q94: Write the balanced chemical equation for the
Q96: A nonpolar bond will form between two
Q97: How many different types of resonance structures
Q112: Electron affinity is a measure of how