Multiple Choice
Which electron configuration denotes an atom in its ground state?
A) 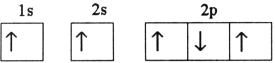
B) 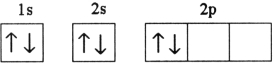
C) 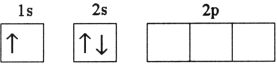
D) 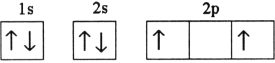
E) 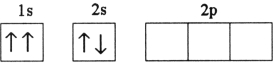
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q39: Which of the subshells below do not
Q40: At maximum, an f- subshell can hold
Q41: The complete electron configuration of gallium, element
Q42: An electron in a Bohr hydrogen atom
Q43: Which electron configuration represents a violation of
Q45: _- orbitals are spherically symmetrical.<br>A) d<br>B) g<br>C)
Q46: The photoelectric effect is .<br>A) the darkening
Q48: The ground state electron configuration of Fe
Q49: Which one of the following represents an
Q156: The ground state electron configuration of copper