Multiple Choice
Which electron configuration represents a violation of the Pauli exclusion principle?
A) 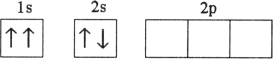
B) 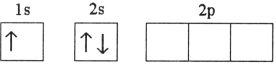
C) 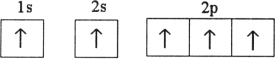
D) 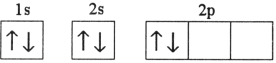
E) 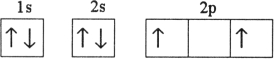
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Blackbody radiation is the emission of light
Q19: When the electron in a hydrogen atom
Q20: In a p<sub>x</sub><sub> </sub>orbital, the subscript x
Q21: Which electron configuration represents a violation of
Q22: The de Broglie wavelength of an electron
Q25: The orbital is degenerate with 5p<sub>y</sub><sub> </sub>in
Q26: A mole of red photons of wavelength
Q28: Of the following, radiation has the shortest
Q29: A mole of yellow photons of wavelength
Q44: The 4d subshell in the ground state