Multiple Choice
Which electron configuration represents a violation of Hund's rule for an atom in its ground state?
A) 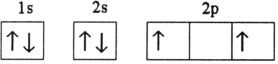
B) 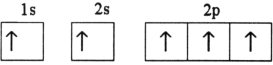
C) 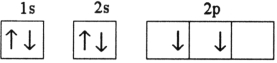
D) 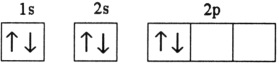
E) 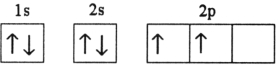
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Which one of the following configurations depicts
Q13: How many quantum numbers are necessary to
Q14: All of the orbitals in a given
Q15: What is the wavelength of light (nm)
Q18: What is the frequency of light (s<sup>-
Q19: When the electron in a hydrogen atom
Q20: In a p<sub>x</sub><sub> </sub>orbital, the subscript x
Q21: Which electron configuration represents a violation of
Q93: The second shell in the ground state
Q158: If a hydrogen atom electron jumps from