Multiple Choice
Which electron configuration represents a violation of the Pauli exclusion principle?
A) 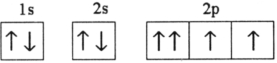
B) 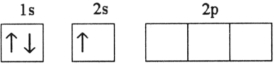
C) 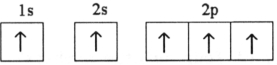
D) 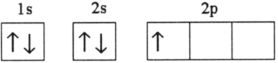
E) 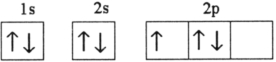
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Elements in group _ have a np<sup>5</sup>
Q29: The largest principal quantum number in the
Q120: An electron cannot have the quantum numbers
Q121: An FM radio station broadcasts electromagnetic radiation
Q122: Of the following, radiation has the shortest
Q123: Which of the subshells below do not
Q124: Which one of the following is the
Q126: Which group in the periodic table contains
Q129: The wavelength of an electron whose velocity
Q130: Which two elements have the same ground-