Multiple Choice
How many moles of magnesium oxide are produced by the reaction of 3.82 g of magnesium nitride with 7.73 g of water? 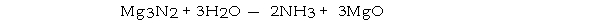
A) 0.0378
B) 0.113
C) 0.429
D) 4.57
E) 0.0756
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The balanced equation for the decomposition of
Q7: A 22.5- g sample of ammonium carbonate
Q8: Pentacarbonyliron (Fe(CO)<sub>5</sub>) reacts with phosphorous trifluoride (PF<sub>3</sub>)
Q9: Calculate the percentage by mass of nitrogen
Q10: There are hydrogen atoms in 25 molecules
Q12: A sample of CH<sub>2</sub>F<sub>2</sub><sub> </sub>with a mass
Q13: There are _ mol of carbon atoms
Q14: When the following equation is balanced, the
Q15: The formula of nitrobenzene is C<sub>6</sub>H<sub>5</sub>NO<sub>2</sub>. The
Q16: What is the coefficient of O<sub>2</sub><sub> </sub>when