Multiple Choice
The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O: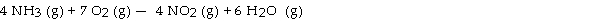
The combustion of 28.8 g of ammonia consumes g of oxygen.
A) 108
B) 94.9
C) 28.8
D) 54.1
E) 15.3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q111: The molecular weight of the acetic acid
Q112: Sulfur and oxygen react in a combination
Q113: What is the empirical formula of a
Q114: What is the empirical formula of a
Q115: When the following equation is balanced, the
Q117: There are _ atoms of oxygen are
Q118: How many oxygen atoms are contained in
Q119: When the following equation is balanced, the
Q120: When the following equation is balanced, the
Q121: The formula weight of aluminum sulfate (Al<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>)