Multiple Choice
Silver nitrate and aluminum chloride react with each other by exchanging anions: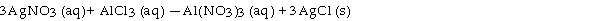
What mass in grams of AgCl is produced when 4.22 g of AgNO3 react with 7.73 g of AlCl3?
A) 11.9
B) 17.6
C) 24.9
D) 3.56
E) 4.22
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q85: Sulfur and fluorine react in a combination
Q86: When the following equation is balanced, the
Q88: Lithium and nitrogen react in a combination
Q89: When the following equation is balanced, the
Q91: When a hydrocarbon burns in air, what
Q92: The formula weight of a substance is
Q93: How many molecules of CH<sub>4</sub><sub> </sub>are in
Q94: When the following equation is balanced, the
Q95: Combustion of a 0.9835- g sample of
Q145: The mass of a single atom of