Multiple Choice
The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O: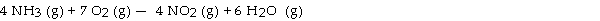
The combustion of 43.9 g of ammonia produces g of NO2.
A) 2.58
B) 178
C) 43.9
D) 0.954
E) 119
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: Of the reactions below, which one is
Q29: A compound is composed of only C,
Q32: When the following equation is balanced, the
Q34: Combustion of a 1.031- g sample of
Q35: When the following equation is balanced, the
Q36: The formula weight of calcium nitrate (Ca(NO<sub>3</sub>)<sub>2</sub>)
Q37: When the following equation is balanced, the
Q38: When the following equation is balanced, the
Q72: The quantity of product that is calculated
Q85: Complete and balance the following reaction, given