Multiple Choice
Calcium carbide (CaC2) reacts with water to produce acetylene (C2H2) :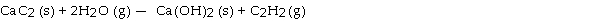
Production of 13g of C2H2 requires consumption of g of H2O.
A) 9.0
B) 18
C) 4.8 × 10- 2
D) 4.8 × 102
E) 4.5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: Write the balanced equation for the reaction
Q102: How many moles of sodium carbonate contain
Q103: What is the empirical formula of a
Q104: A compound contains 38.7% K, 13.9% N,
Q105: Solid aluminum and gaseous oxygen react in
Q107: When the following equation is balanced, the
Q108: A 2.25- g sample of magnesium nitrate,
Q109: When the following equation is balanced, the
Q110: What is the mass % of carbon
Q111: The molecular weight of the acetic acid