Multiple Choice
Based on entropy considerations alone, which homogeneous aqueous equilibrium would be expected to lie to the right?
A) AgI2- + 2Br- 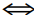 AgBr2- + 2I-
AgBr2- + 2I-
B) Fe(NH3) 62+ + C20H10N42- 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Fe(NH3) 2(C20H10N4) + 4NH3
C) CoCl42+ + 6H2O 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Co(H2O) 62+ + 4Cl-
D) Ni(H2NC2H4NH2) 32+ + 6NH3 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Ni(NH3) 62+ + 3H2NC2H4NH2
E) Cu(NH3) 42+ + 6H2O 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Cu( H2O) 62+ + 4NH3
Correct Answer:

Verified
Correct Answer:
Verified
Q5: How can high-spin and low-spin transition metal
Q34: Define the chelate effect.
Q65: Does either or both cis- or trans-[Mn(en)<sub>2</sub>Br<sub>2</sub>]
Q94: What is the purpose of adding EDTA
Q96: What are the donor atoms in ferrichrome
Q97: Based on electron configuration, which is most
Q101: How many d electrons are in the
Q102: Metals with _ electron configurations characteristically form
Q103: Based on the crystal- field strengths F<sup>-
Q140: Werner's theory of primary and secondary valences