Multiple Choice
Which of the following equations represents the roasting of an ore?
A) All of these represent roasting processes.
B) PbO (s) + CO (g) 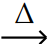 Pb (l) + CO2 (g)
Pb (l) + CO2 (g)
C) HgS (s) + O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Hg (g) + SO2 (g)
D) 2ZnS (s) + 3O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_112ZnO (s) + 2SO2 (g)
E) 2MoS2 (s) + 7O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_112MoO3 (s) + 4SO2 (g)
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Of the following, which is a bulk
Q32: What is the coefficient of Fe<sup>2</sup><sup>+ </sup>when
Q33: The Hall process is<br>A) the pyrometallurgic process
Q34: Write the two reactions that are used
Q35: The hydrated manganese(II) ion is<br>A) blue.<br>B) violet.<br>C)
Q37: In the second and third transition series,
Q38: The purpose of a converter in steel
Q39: Alloys generally differ from compounds in that<br>A)
Q40: What two oxidation states are more frequently
Q41: The respective standard oxidation potentials for Cu