Multiple Choice
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the binding energy (in J) of a 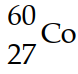 nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.)
nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.)
A) 8.206 × 10- 11
B) 9.117 × 10- 28
C) 4.940 × 10- 13
D) 2.735 × 10- 16
E) 2.735 × 10- 19
Correct Answer:

Verified
Correct Answer:
Verified
Q114: By what process does thorium- 230 decay
Q115: Bombardment of uranium- 235 with a neutron
Q117: Which of the following correctly represents the
Q118: On average, neutrons are produced by every
Q120: Radium undergoes alpha decay. The product of
Q121: Strontium- 90 is a byproduct in nuclear
Q123: Which type of radioactive decay results in
Q124: In radioactive dating,the ratio of carbon-12 to
Q124: What is the atomic number of a
Q129: What order process is radioactive decay?<br>A)zeroth<br>B)first<br>C)second<br>D)third<br>E)fourth