Multiple Choice
The standard cell potential (E°cell) for the voltaic cell based on the reaction below is V. 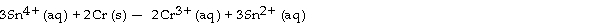
A) +0.89
B) - 0.59
C) - 1.02
D) +1.94
E) +2.53
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: What is the oxidation number of manganese
Q19: Which transformation could take place at the
Q20: The standard cell potential (E°) of a
Q21: How many grams of Ca metal are
Q22: The lead- containing reactant(s) consumed during recharging
Q24: The electrolysis of molten AlCl<sub>3 </sub>for 3.25
Q25: The standard cell potential (E°<sub>cell</sub>) of the
Q26: is the oxidizing agent in the reaction
Q27: Which substance is serving as the reducing
Q56: The anode of the alkaline battery is