Multiple Choice
The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is (Amu) 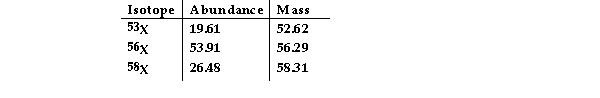
A) 57.23
B) 56.11
C) 33.33
D) 56.29
E) 55.74
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q131: Which one of the following does not
Q132: The average atomic weight of copper, which
Q133: Which of the following compounds would you
Q134: Which type of formula provides the most
Q135: Which species below is the nitride ion?<br>A)
Q137: The correct name for CaH<sub>2</sub><sub> </sub>is _
Q138: Which formula/name pair is incorrect? <br>A) <img
Q139: Which pair of elements would you expect
Q140: _- rays consist of fast- moving electrons.<br>A)
Q141: The nucleus of an atom does not