Multiple Choice
Silver has two naturally occurring isotopes with the following isotopic masses: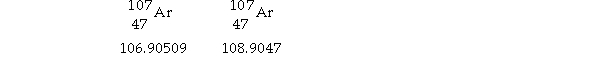
The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is .
A) 0.7578
B) 0.9047
C) 0.5184
D) 0.2422
E) 0.4816
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: A molecular formula always indicates .<br>A) the
Q21: The suffix - ide is used .<br>A)
Q22: The mass number of an atom of
Q24: 200 pm is the same as Å.<br>A)
Q26: Which one of the following is not
Q27: Gravitational forces act between objects in proportion
Q28: Potassium is a _ and chlorine is
Q29: Lithium is a and magnesium is a
Q30: The element X has three naturally occurring
Q64: The formula for chromium (II)iodide is CrI<sub>2</sub>.