Multiple Choice
The element X has two naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is amu. 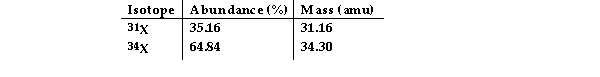
A) 34.02
B) 30.20
C) 35.22
D) 32.73
E) 33.19
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q162: Of the following, the smallest and lightest
Q163: the possible oxication numbers for gold are
Q164: Which one of the following species has
Q165: The name of PCl<sub>3</sub><sub> </sub>is _ .<br>A)
Q166: The charge on the manganese in the
Q168: Which element forms an ion with the
Q169: Which pair of elements below should be
Q170: In the periodic table, the elements are
Q171: Predict the empirical formula of the ionic
Q172: Which species below is the nitrate ion?<br>A)