Multiple Choice
Consider the following table of Ksp values. 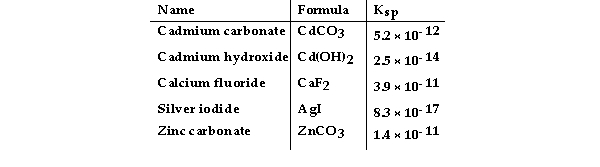
-Which compound listed below has the greatest molar solubility in water?
A) CdCO3
B) AgI
C) ZnCO3
D) CaF2
E) Cd(OH) 2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Which of the following could be added
Q21: Consider a solution containing 0.100 M fluoride
Q22: Suppose you have just added 100.0 ml
Q23: Calculate the pH of a solution that
Q24: Calculate the pH of a solution prepared
Q26: A 25.0 mL sample of 0.723 M
Q27: Which of the following could be added
Q28: Which one of the following will cause
Q29: A 25.0 mL sample of a solution
Q30: Consider a solution containing 0.100 M fluoride