Multiple Choice
Consider the following table of Ksp values. 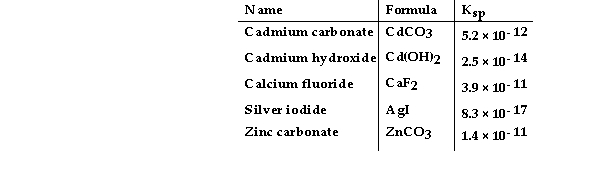
-Which compound listed below has the smallest molar solubility in water?
A) CdCO3
B) CaF2
C) Cd(OH) 2
D) ZnCO3
E) AgI
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q76: The pH of a solution prepared by
Q77: A 25.0 mL sample of 0.723 M
Q78: A 25.0- mL sample of 0.150 M
Q79: Calculate the pH of a buffer solution
Q80: Determine the K<sub>sp</sub><sub> </sub>for magnesium hydroxide (Mg(OH)<sub>2</sub>)
Q82: 200.0 ml of a solution containing 0.5000
Q83: What is the pH of a buffer
Q84: What is the molar solubility of magnesium
Q85: How many milliliters of 0.0850 M NaOH
Q86: Suppose you have just added 200.0 ml