Multiple Choice
In which of the following reactions would increasing pressure at constant temperature not change the concentrations of reactants and products, based on Le Cha^telier's principle?
A) 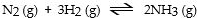
B) 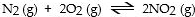
C) 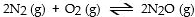
D) 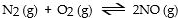
E) 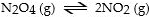
Correct Answer:

Verified
Correct Answer:
Verified
Q15: The K<sub>eq </sub>for the equilibrium below is
Q15: The relationship between the concentrations of reactants
Q16: The effect of a catalyst on an
Q17: The K<sub>eq</sub><sub> </sub>for the equilibrium below is
Q19: Le Chatelier's principle states that if a
Q21: Consider the following reaction at equilibrium:<br>2SO<sub>2 </sub>(g)
Q23: Consider the following reaction at equilibrium:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg"
Q24: Acetic acid is a weak acid that
Q25: The reaction below is exothermiC: 2SO<sub>2 </sub>(g)
Q49: At constant temperature, reducing the volume of