Multiple Choice
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) →ClO3- (aq) + ClO2- (aq) + H2O (1) 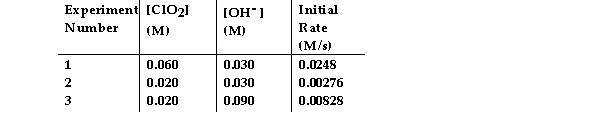
-What is the order of the reaction with respect to ClO2?
A) 0
B) 2
C) 1
D) 3
E) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: Heterogeneous catalysts have different phases from reactants.
Q54: The reaction A - B is first
Q55: The rate law of the overall reaction
Q56: The reaction A - B is first
Q57: Nitrogen fixation is a difficult process because
Q58: Of the following, will lower the activation
Q60: As the temperature of a reaction is
Q62: The reaction<br>2NO<sub>2 </sub>→ 2NO + O<sub>2</sub><br>Follows second-
Q63: At elevated temperatures, dinitrogen pentoxide decomposes to
Q64: The average rate of disappearance of I<sup>-</sup><sup>