Multiple Choice
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1) 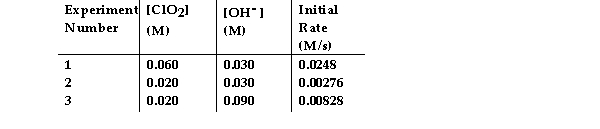
-What is the order of the reaction with respect to OH- ?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer:

Verified
Correct Answer:
Verified
Q26: The primary source of the specificity of
Q28: The isomerization of methylisonitrile to acetonitrile CH<sub>3</sub>NC
Q30: The elementary reaction<br>2NO<sub>2 </sub>(g) → 2NO (g)
Q32: Reaction rates are affected by reactant concentrations
Q32: If the rate law for the reaction
Q33: At elevated temperatures, methylisonitrile (CH<sub>3</sub>NC) isomerizes to
Q34: Consider the following reaction:<br>3A - 2B<br>The average
Q35: The rate of a reaction depends on
Q36: A flask is charged with 0.124 mol
Q92: The relationship of absorbed light to the