Multiple Choice
The data in the table below were obtained for the reaction:
A + B → P 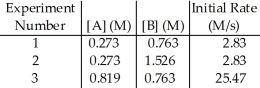
-The order of the reaction in B is ________.
A) 1
B) 2
C) 3
D) 4
E) 0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: The reaction 2NOBr (g)→ 2 NO (g)+
Q117: The overall order of a reaction is
Q120: Nitrogen fixation is a difficult process because
Q122: Consider the following reaction: A → 2C<br>The
Q123: A compound decomposes by a first-order process.
Q124: At elevated temperatures, dinitrogen pentoxide decomposes to
Q126: The uptake of molecules into the interior
Q128: A first-order reaction has a rate constant
Q128: The Earth's ozone layer is located in
Q130: The rate constant for a particular second-order