Multiple Choice
Which one of the following substances will not have hydrogen bonding as one of its intermolecular forces?
A) 
B) 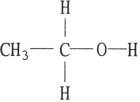
C) 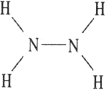
D) 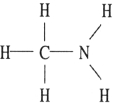
E) 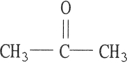
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: The predominant intermolecular force in (CH<sub>3</sub>)<sub>2</sub>NH is
Q3: The unit cell with all sides the
Q3: The phase diagram of a substance is
Q4: Elemental iodine (I<sub>2</sub>) is a solid at
Q5: The phase changes B -C and D
Q6: Metallic solids do not exhibit .<br>A) extreme
Q8: The substance with the largest heat of
Q9: A volatile liquid is one that .<br>A)
Q11: Which of the following molecules has hydrogen
Q81: Chromium crystallizes in a body-centered cubic unit