Multiple Choice
Based on molecular mass and dipole moment of the five compounds in the table below, which should have the highest boiling point? 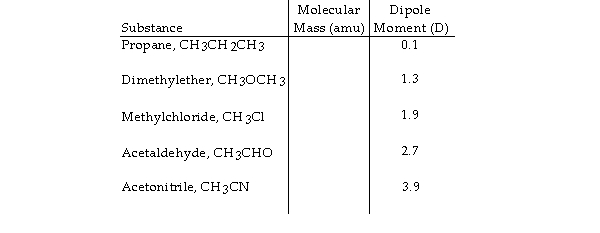
A) CH3OCH3
B) CH3CHO
C) CH3CN
D) CH3CH2CH3
E) CH3Cl
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: The type of solid that is characterized
Q26: A substance that expands to fill its
Q28: The shape of a liquid's meniscus is
Q29: The strongest interparticle attractions exist between particles
Q30: What fraction of the volume of each
Q32: Viscosity is .<br>A) the same as density<br>B)
Q33: is the energy required to expand the
Q34: The phase diagram of a substance is
Q35: Consider the following statements about crystalline solids:<br>(i)
Q36: Crystalline solids differ from amorphous solids in