Multiple Choice
A gas vessel is attached to an open- end manometer containing a nonvolatile liquid of density 0.791 g/mL as shown below.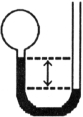
The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mmHg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is atm.
A) 0.993
B) 1.03
C) 0.987
D) 0.960
E) 0.990
Correct Answer:

Verified
Correct Answer:
Verified
Q129: The density of nitric oxide (NO) gas
Q130: If 3.21 mol of a gas occupies
Q131: The reaction of 50 mL of N<sub>2</sub><sub>
Q132: CO (5.00 g) and CO<sub>2</sub><sub> </sub>(5.00 g)
Q133: Which statement about atmospheric pressure is false?<br>A)
Q135: What is the density (in g/L) of
Q136: A sample of H<sub>2</sub><sub> </sub>gas (12.28 g)
Q137: The molecular weight of a gas that
Q139: At a temperature of _ °C, 0.444
Q159: Kinetic-molecular theory assumes that attractive and repulsive