Multiple Choice
Which of the following statements is true concerning the voltaic cell shown below? 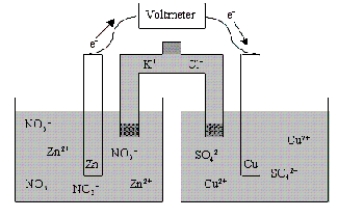
A) The Zn anode mass decreases as the cell discharges.
B) The Zn cathode mass increases as the cell discharges.
C) The Zn cathode mass decreases as the cell discharges.
D) The Zn anode mass increases as the cell discharges.
E) The mass of the Zn electrode neither increases nor decreases as the cell discharges.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Calculate Δ<sub>r</sub>G° for the disproportionation reaction of
Q5: Which of the following statements is true
Q24: Write a balanced half-reaction for the
Q25: For the following cell reaction, the
Q31: Which of the following statements concerning voltaic
Q32: When the given oxidation-reduction reaction in an
Q45: When a secondary battery provides electrical energy,it
Q50: Calculate the charge,in coulombs,is required to deposit
Q66: Consider the following half-reactions. Ag<sup>+</sup>(aq)+ e<sup>-</sup> →
Q78: Which of the following is true for