Multiple Choice
What is the simplest formula of the compound represented by the unit cell provided below? 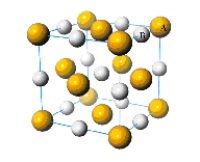
A) AB
B) AB2
C) AB3
D) A2B3
E) A2B4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Which of the following statements is true
Q11: Which of the following compounds is expected
Q18: In metals,there are not enough electrons to
Q24: A metal crystallizes in a face-centered cubic
Q25: According to the below phase diagram, what
Q31: Point D on the phase diagram is
Q32: What is the length of the
Q47: If an ionic compound with the formula
Q50: Nickel has a face-centered cubic cell,and its
Q58: Strontium oxide has a face centered cubic