Multiple Choice
A sketch of a phase diagram is given below. 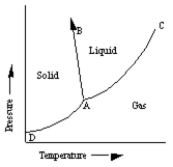 Which statement about this diagram is not true?
Which statement about this diagram is not true?
A) Increasing pressure at constant temperature can melt the solid.
B) Increasing temperature at constant pressure can cause the solid to sublime.
C) Increasing temperature at constant pressure can cause the liquid to vaporize.
D) Increasing pressure at constant temperature can cause deposition of solid from gas.
E) Increasing pressure at constant temperature can cause liquid to freeze.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: The bandgap of ZnTe is 218 kJ/mol.What
Q30: Silver chloride adopts the sodium chloride (rock
Q32: A metal crystallizes in a face-centered cubic
Q49: A low-melting solid readily dissolves in water
Q59: Using the following data to calculate
Q59: Which process requires the greatest endothermic change
Q60: On the phase diagram below, which point
Q63: Which equation represents the number of
Q66: What is the distance, in atomic
Q67: In what type of unit cell are