Multiple Choice
How many sigma (σ) bonds and pi (π) bonds are present in the given molecule? 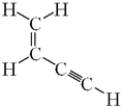
A) Seven σ and three π
B) Seven σ and two π
C) Five σ and five π
D) Five σ and three π
E) Five σ and two π
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the hybridization of the central
Q27: Refer to Diagram 9-1.Assume that the molecular
Q30: How many sigma (σ)bonds and pi (π)bonds
Q35: Refer to diagram 9-1.Identify the molecule with
Q41: What is the hybridization of the sulfur
Q43: Refer to Diagram 9-1.What is the molecular
Q51: In HF<sub>2</sub>− the hydrogen is shared between
Q53: Refer to Diagram 9-1.According to molecular orbital
Q55: What does the following figure represent? <img
Q57: Which molecule will have the following valence