Multiple Choice
Which of the underlined atoms (C1, C2, N, and O) are sp2 hybridized? 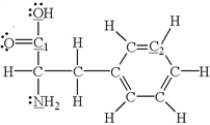
A) N and O
B) C1 and N
C) C1 and O
D) C2 and N
E) C1 and C2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: What is the molecular geometry around an
Q7: A molecular orbital that decreases the electron
Q11: Refer to Diagram 9-1.According to molecular orbital
Q21: Which diatomic molecule or ion has valence
Q24: The electron configuration of a particular diatomic
Q28: What is the molecular geometry around a
Q31: If 7 orbitals on one atom overlap
Q37: In molecular orbital theory,the bond order is
Q46: SF<sub>3</sub><sup>+</sup>,has a tetrahedral electron-pair geometry and a
Q48: Benzene,C<sub>6</sub>H<sub>6</sub>,consists of a six member ring of