Multiple Choice
What is the formal charge on the O2 atom in the following Lewis structure? 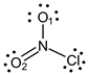
A) 2
B) 1
C) 0
D) -1
E) -2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: Determine the bond order of NO+?<br>A) 2<br>B)
Q25: The central atom in N<sub>2</sub>O is a
Q39: Electronegativity is a measure of _.<br>A) the
Q49: Use VSEPR theory to predict the electron-pair
Q54: What is the formal charge on the
Q59: Which of the following molecules is polar?<br>A)
Q69: What is the correct Lewis structure for
Q71: According to VSEPR theory,which of the following
Q74: Use Lewis structures to predict the bond
Q77: Which of the following is the correct