Multiple Choice
What is the formal charge of the oxygen atom in the Lewis structure for cyanate shown below? 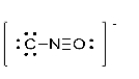
A) 0
B) −2
C) +1
D) −1
E) 2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Three possible structures of C<sub>2</sub>H<sub>2</sub>Cl<sub>2</sub> are shown
Q4: Based on electron-pair geometries,which of the following
Q9: Which of the following Lewis structures for
Q10: Which of the following species has the
Q11: Which of the following are the correct
Q14: Which of the following groups of molecules
Q26: Use VSEPR theory to predict the molecular
Q34: Use VSEPR theory to predict the electron-pair
Q65: The rings in the nitrogen-containing bases in
Q77: Which of the following has a Lewis