Multiple Choice
Three nonequivalent Lewis structures for carbonyl sulfide, SCO, are given below. Use the concepts of formal charge and electronegativity to choose the structure that is the best representation. 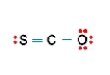 A
A 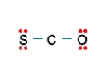 B
B 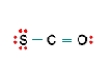 C
C
A) Structure A, because all the formal charges equal 0
B) Structure B, because all the formal charges equal 0
C) Structure C, because all the formal charges equal 0.
D) Structure A, because the negative formal charge resides on the most electronegative atom
E) Structure C, because the negative formal charge resides on the most electronegative atom
Correct Answer:

Verified
Correct Answer:
Verified
Q2: According to VSEPR theory,which of the following
Q32: Which of the following molecules or ions
Q35: How many valence electrons are present in
Q38: One might expect PCl<sub>3</sub> to have a
Q58: Which of the following elements is most
Q65: Using bond-energy data, what is ?<sub>r</sub>H
Q73: Linus Pauling noticed that the energy of
Q76: What is the molecular geometry around an
Q78: Use VSEPR theory to predict the molecular
Q83: Place the following molecules in order from