Multiple Choice
For which of the following electron transitions would a hydrogen atom emit a photon of the longest wavelength?
A) 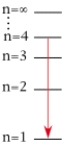
B) 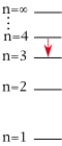
C) 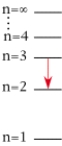
D) 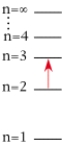
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: A 4d orbital has ?<br>A) 3 planar
Q36: The contribution for which de Broglie is
Q40: What is the energy per mole of
Q43: If a cordless phone operates at a
Q47: The size of an electron orbital is
Q52: Which of the following is the correct
Q55: A device emits light at 219.4 nm.What
Q57: Occupied states or energy levels in the
Q58: A device operates at a frequency
Q59: Which type of orbital is designated