Multiple Choice
When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) by the following reaction, 98.8 kJ of energy is absorbed.
Fe2O3(s) + 3 H2(g) → 2 Fe(s) + 3 H2O(g) 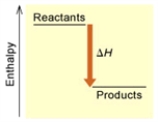 (A)
(A) 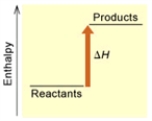
(B)
Is the reaction endothermic or exothermic, and which of the enthalpy diagrams above
Represents this reaction?
A) Endothermic; A
B) Endothermic; B
C) Exothermic; A
D) Exothermic; B
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The heat required to convert a solid
Q20: The standard molar enthalpy of formation of
Q22: Determine the heat of evaporation of
Q23: Determine the standard enthalpy of formation
Q23: Calculate the energy in the form of
Q24: Given the thermochemical equation <br>4AlCl<sub>3</sub>(s) +
Q36: Why are you at greater risk from
Q40: Internal energy and enthalpy are state functions.What
Q46: CaO(s)reacts with water to form Ca(OH)<sub>2</sub>(aq).If 6.50
Q62: At constant pressure and 25°C,what is Δ<sub>r</sub>H°