Multiple Choice
The mass spectrum of an element with two naturally occurring isotopes is shown below. What is the best estimate of the element's (average) atomic weight? 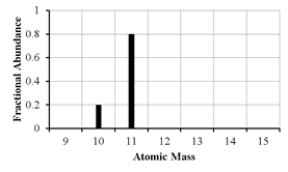
A) 10 amu
B) 11 amu
C) 10.8 amu
D) 10.2 amu
E) 10.5 amu
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: An ionic compound has the formula MCl<sub>2</sub>.The
Q20: How many protons are there in an
Q36: Calculate the mass percent of chlorine in
Q42: Which of the following elements belongs to
Q46: For a nonmetal in Group 6A of
Q50: William Crookes was the first to observe
Q62: What is the symbol for an ion
Q73: The formula for aluminum fluoride is _.<br>A)
Q76: Which of the following is the correct
Q77: Calculate the number of moles of aluminum