Multiple Choice
The chair and boat conformations of cyclohexane are shown below.Which statement best explains why the chair conformation is more stable than the boat conformation? 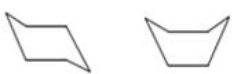
A) The hydrogen atoms in the chair conformation are all staggered,which minimizes the repulsion of the bonding electrons.
B) The carbon atoms in the chair conformation are all eclipsed,which provides for the ideal bond angle of 109.5°.
C) The carbon atoms in the chair conformation are closer together than in the boat conformation,which provides for stronger C-C bonds.
D) The hydrogen atoms in the chair conformation are closer together than in the boat conformation,which provides for stronger C-H bonds.
E) The C-C bonds in the chair conformation are all staggered,which maximizes the energy of the molecule.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: What is the IUPAC name of the
Q2: An alkane used as a lubricant contains
Q3: How does the molecular formula of a
Q5: Which of the following best describes molecules
Q6: What is the IUPAC name of the
Q7: Benzene may be represented by the line
Q8: Which compound(s)below contain substituents that are trans?
Q9: Butanone (structure shown)may be classified as what
Q10: Which of the following statements concerning conformational
Q11: The propyl group is CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>-.